Each patient's genetic background is unique, and genetic testing provides an important basis for personalized medicine, making medical decisions more precisely targeted at individuals rather than based on the average response of the group. This is mainly reflected in:
● Precise drug selection
Through genetic testing, we can understand the genetic characteristics of drug-metabolizing enzymes, drug targets, etc. in the patient's body, and thus screen out the most suitable drugs for the patient.
● Optimizing drug dosage
Based on the patient's genetic information, their metabolic rate and sensitivity to drugs can be predicted, and the drug dosage can be adjusted reasonably.
● Predicting serious adverse reactions
Identify gene variants that are highly associated with serious adverse reactions to specific drugs in advance to conduct pre-drug risk assessments. For example, patients carrying the HLA-B*1502 allele have a significantly increased risk of Stevens-Johnson syndrome/toxic epidermal necrolysis when using carbamazepine. Genetic testing can screen for this gene variant in advance and avoid the use of carbamazepine.
● Reduce ineffective treatments and common adverse reactions
Through genetic testing, precise medication can be achieved, making drugs more effective faster, shortening the treatment cycle, reducing medical expenses and patients' living costs caused by long-term treatment, and promoting the development of personalized medicine.
Pharmacogenomic testing has been widely used in multiple disease areas to guide the safe and effective use of specific drugs. The following are common test categories and representative gene loci:
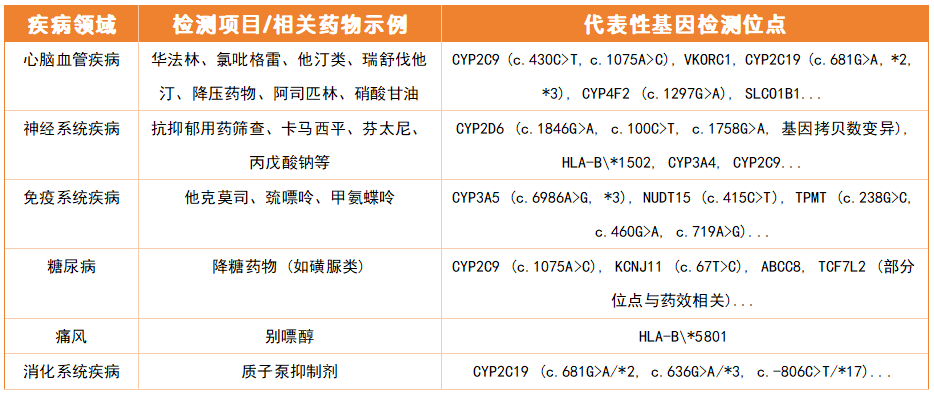
Note: The above are typical high-frequency application scenarios. Please scan the QR code to contact an exclusive consultant for a complete list.
Project Background:
A client needed to develop a multi-SNP detection kit based on probe-based qPCR technology for comprehensive disease risk assessment and medication guidance for hospitalized patients. To optimize costs and address quality issues with the existing solution (the original supplier's solution had frequently generated quality complaints from clients), the client outsourced the project to Qihengxing.
Qihengxing solutions and results:
After taking over, the Qihengxing team conducted a comprehensive reassessment of the project's technical route and developed a new R&D plan accordingly. After a year of rigorous demonstration, experimental optimization, and verification, they ultimately achieved significant improvements in key performance indicators:
Gray Zone Detection: Significantly reduced from approximately 10% to<2%,大幅提高分型结果的确定性。
Amplification fluorescence increment (Rn): An increase from 1-2 to 2.5-4.5 indicates higher amplification efficiency and stronger signal.
Ct value difference of heterozygous samples (ΔCt): The reduction from 2.0 to <0.5 significantly improved the accuracy and readability of heterozygote typing.
Typing interpretation: The overall typing results are clearer and more accurate, and the accuracy of interpretation is significantly improved.
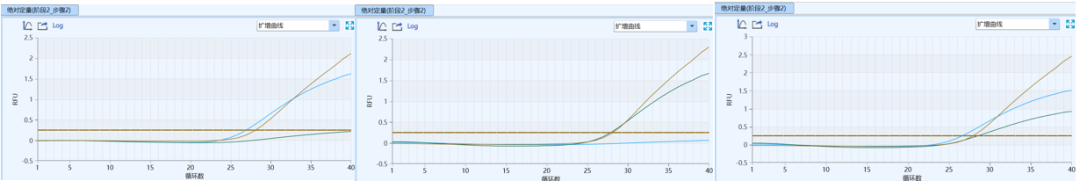
Figure 1 Amplification curves of wild-type, mutant, and heterozygous samples at the initial R&D stage of the kit
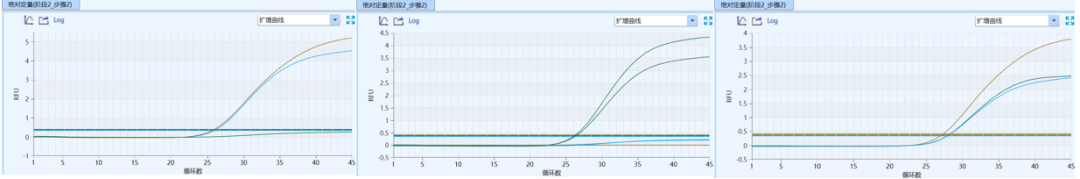
Figure 2 Amplification curves of wild-type, mutant, and heterozygous samples at the late-stage kit site
Project Results:
Qihengxing successfully completed the research and development of the test kit and achieved stable production, and has been providing customers with continuous and stable supply. 2 years During this period, the product "Zero" quality accidents The record of Qihengxing fully verifies its excellent stability and reliability. Based on the high-quality delivery of this project, Qihengxing has won the deep trust of the customer. Renew new project technology entrustment development contract.